Eye Drops Recall: FDA Warns Of Contamination Risk
Editor's Notes: Eye Drops Recall: FDA Warns Of Contamination Risk have been published on today date, Stating the risk of contamination of certain eye drops. A recall has been issued for several batches of EzriCare Artificial Tears due to potential contamination with the bacteria Pseudomonas aeruginosa. The FDA warns consumers not to use the affected eye drops and to seek medical attention immediately if they experience any symptoms after using them.
Our team has analyzed this situation and dug up the information to help you understand the potential risks and what you can do. This guide will provide you with the key takeaways of the Eye Drops Recall: FDA Warns Of Contamination Risk.
FAQ
The U.S. Food and Drug Administration (FDA) is warning consumers and healthcare professionals about the potential risk of contamination with _Pseudomonas aeruginosa_ in EzriCare Artificial Tears. The FDA advises against using the artificial tears and is recommending that the product be discarded. The FDA is also investigating the matter and working with the company to address the contamination issue. Eye Drops Recall: FDA Warns Of Contamination Risk

2023 FDA Eye Drop Recall: Consumer Safety Information - La Jolla, CA - Source www.gweye.com
Question 1: What is the risk of contamination with EzriCare Artificial Tears?
Pseudomonas aeruginosa is a type of bacteria that can cause serious infections in the eyes, including corneal ulcers, which can lead to blindness. The FDA has received reports of eye infections, including corneal ulcers, in patients who have used EzriCare Artificial Tears.
Question 2: What are the symptoms of an eye infection caused by _Pseudomonas aeruginosa_?
Symptoms may include eye pain, redness, swelling, discharge, and vision problems.
Question 3: Who is most at risk of an eye infection from EzriCare Artificial Tears?
Anyone who has used EzriCare Artificial Tears is at risk of an eye infection. However, people who have a weakened immune system, wear contact lenses, or have other eye conditions may be at a higher risk.
Question 4: What should I do if I have used EzriCare Artificial Tears?
If you have used EzriCare Artificial Tears, stop using the product immediately and discard it. Contact your healthcare provider if you have any eye pain, redness, swelling, discharge, or vision problems.
Question 5: What is the FDA doing to address the contamination issue?
The FDA is investigating the matter and working with the company to address the contamination issue. The FDA is also recommending that consumers and healthcare professionals stop using EzriCare Artificial Tears and discard the product.
Question 6: Where can I find more information about the EzriCare Artificial Tears recall?
You can find more information on the FDA's website.
The FDA encourages consumers and healthcare professionals to report any adverse events related to EzriCare Artificial Tears to the FDA's MedWatch Adverse Event Reporting program.
Tips
The FDA has issued a recall for certain eye drops due to potential contamination. If you have used these drops, it is important to take the following precautions:
Tip 1: Stop using the affected eye drops immediately.
Tip 2: Contact your healthcare provider for further instructions.
Tip 3: Discard any remaining eye drops.
Tip 4: Wash your hands thoroughly with soap and water.
Tip 5: Monitor your eyes for any signs of infection, such as redness, swelling, or discharge.
Eye Drops Recall: FDA Warns Of Contamination Risk
The U.S. Food and Drug Administration (FDA) recently announced a voluntary recall of certain eye drops due to potential contamination. Six key aspects concerning this issue include: contaminated preservative, potential infection, precautionary measures, specific products affected, investigation ongoing, and consumer safety.
- Contaminated preservative: The eye drops are contaminated with the bacteria Pseudomonas aeruginosa, which can cause serious infections.
- Potential infection: The contamination poses a risk of infection to the eyes, including corneal ulcers, endophthalmitis, and even blindness.
- Precautionary measures: The FDA advises consumers to stop using the affected eye drops and consult with a healthcare professional if they have any concerns about potential exposure.
- Specific products affected: The recall affects certain batches of Artificial Tears Lubricant Eye Drops and Artificial Tears Plus Lubricant Eye Drops manufactured by EzriCare LLC, in both single-use vials and multi-dose bottles.
- Investigation ongoing: The FDA is investigating the source of the contamination and working with the manufacturer to ensure the safety of future products.
- Consumer safety: The FDA emphasizes the importance of using sterile and properly stored eye drops to prevent contamination and potential health risks.
This eye drops recall highlights the crucial aspects of product safety, potential health risks associated with contamination, the importance of precautionary measures, and the need for ongoing investigations to safeguard consumer well-being.

FDA Warns Consumers About Eye Drops Recall Over Infection Risks - Source www.consumernotice.org
Eye Drops Recall: FDA Warns Of Contamination Risk
On January 25, 2023, the U.S. Food and Drug Administration (FDA) issued a nationwide recall of EzriCare Artificial Tears due to potential contamination with the bacteria Pseudomonas aeruginosa. This bacteria can cause serious eye infections, including corneal ulcers, which can lead to blindness. The recall includes all lots of EzriCare Artificial Tears, which were distributed to retail stores and online retailers nationwide.
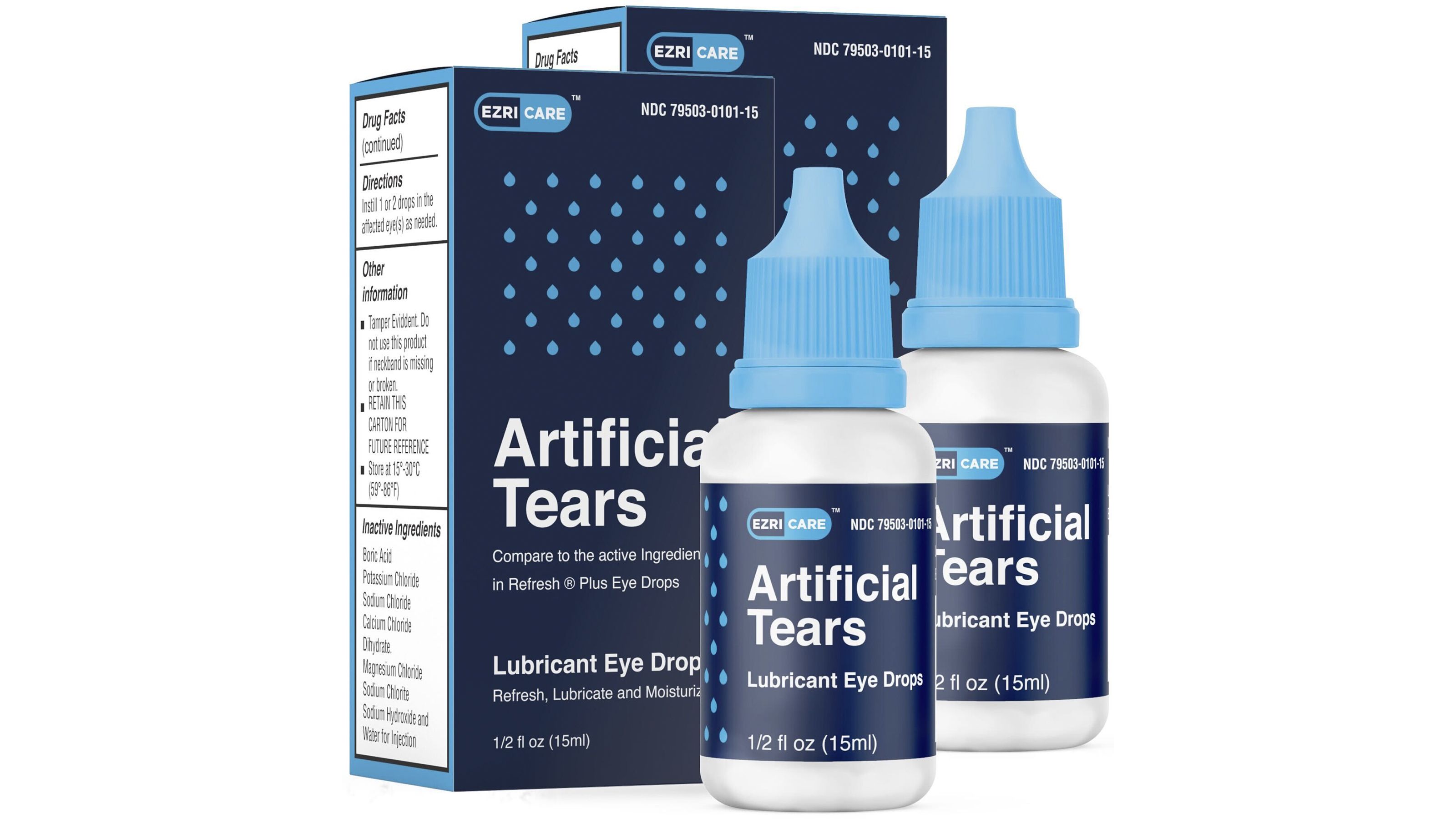
Eye Drops Recall 2024 List Walmart - Audra Candide - Source ivoryqjennie.pages.dev
The FDA's investigation into the contamination is ongoing, but the agency believes that the bacteria may have been introduced during the manufacturing process. EzriCare has voluntarily recalled all lots of its Artificial Tears products and is working with the FDA to investigate the cause of the contamination.
Patients who have used EzriCare Artificial Tears are urged to stop using them immediately and to discard any remaining product. They should also see a doctor if they experience any eye pain, redness, swelling, or discharge.
The FDA is continuing to investigate the contamination and is working with EzriCare to ensure that all affected products are removed from the market.
| EzriCare Artificial Tears have been recalled due to potential contamination with Pseudomonas aeruginosa. | This bacteria can cause serious eye infections, including corneal ulcers, which can lead to blindness. |
| The FDA is investigating the cause of the contamination, but believes it may have been introduced during the manufacturing process. | EzriCare has voluntarily recalled all lots of its Artificial Tears products and is working with the FDA to investigate the cause of the contamination. |
| Patients who have used EzriCare Artificial Tears are urged to stop using them immediately and to discard any remaining product. | They should also see a doctor if they experience any eye pain, redness, swelling, or discharge. |
| The FDA is continuing to investigate the contamination and is working with EzriCare to ensure that all affected products are removed from the market. | Consumers should be aware of this recall and take appropriate action to protect their health. |
Conclusion
The recall of EzriCare Artificial Tears is a reminder of the importance of using only FDA-approved eye care products. Consumers should be aware of the risks associated with using contaminated eye drops and should take steps to protect their health.
The FDA is committed to ensuring the safety of eye care products and will continue to investigate this recall and take appropriate action to protect the public health.